Ulcerative Colitis
A chronic disease with significant unmet need
Requiring treatment of the underlying cause, not just symptoms
Mild-to-moderate ulcerative colitis
A chronic, autoimmune, inflammatory bowel disease

MH002 – a novel treatment strategy in IBD
Immune modulation and restoration
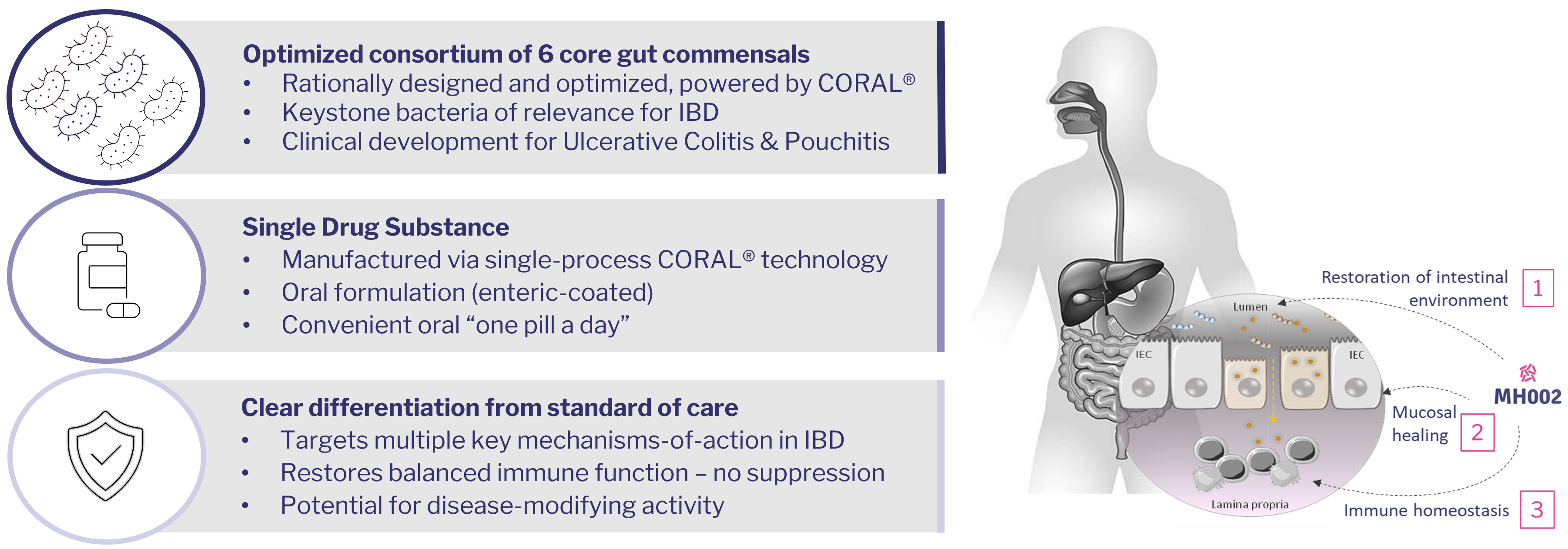
The most advanced rationally-designed consortium therapy for Ulcerative Colitis
MH002 – our lead clinical program
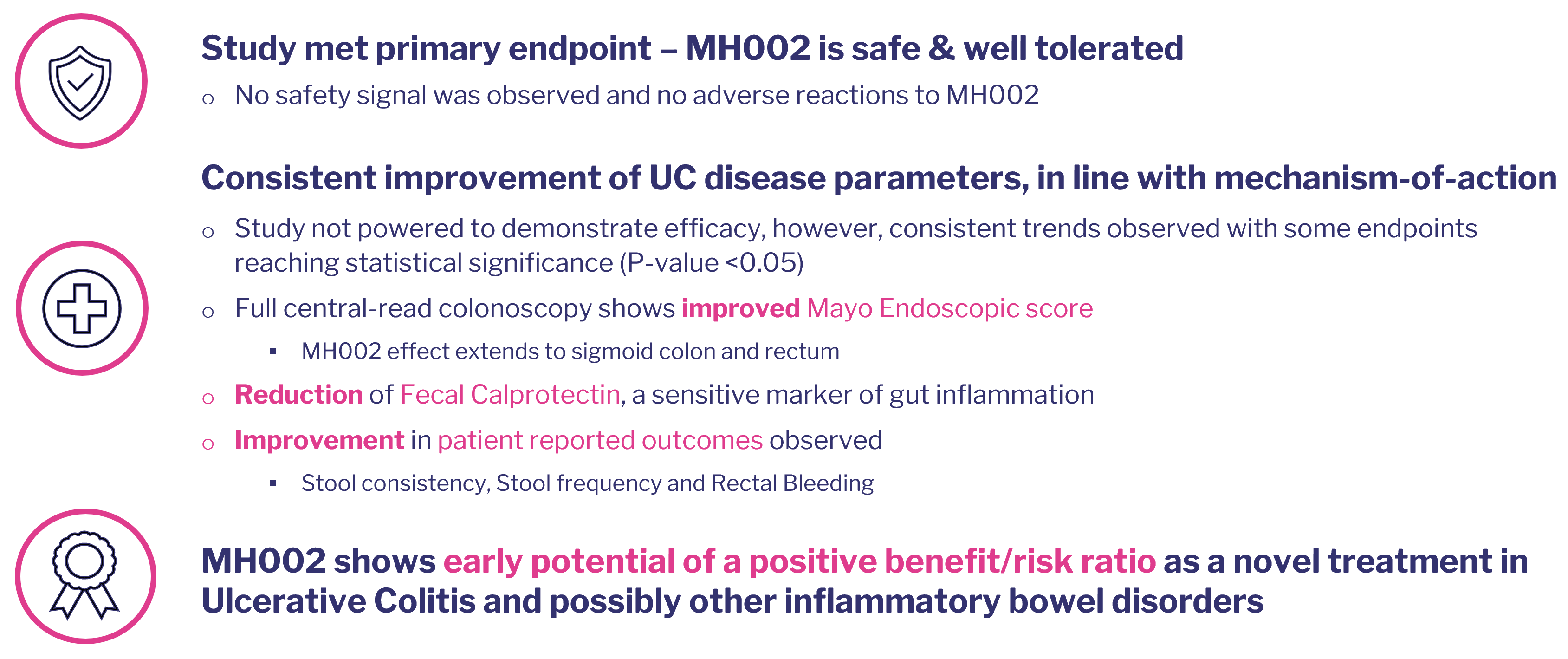
A multi-center, double-blind, randomized, placebo-controlled trial has been completed in 45 UC patients at multiple clinical sites in Belgium, Poland and Czech Republic.
The trial aimed to evaluate safety (primary endpoint), initial efficacy and mechanistic effects of MH002 over eight weeks, with a further eight-week extension period.
More information on the Phase 2a trial design